

When a reaction occurs or a piston moves, all else is not equal, and other sources of entropy production/depletion must be considered.
SPONTANEITY THERMODYNAMICS FREE
When we wrote the Gibbs Free Energy formula, we said change in G is equal to delta H minus T times delta. And at this point, this should look reasonably familiar to you. This is true for any irreversible, spontaneous process. Which means, since $C_p>0$ that, yes, as you state the entropy goes down if the temperature drops, all else being equal. The heat added to the irreversible process minus T times delta S of the irreversible process is going to be less than 0. The Second Law of Thermodynamics states: For any spontaneous process in a closed system the entropy of the system must increase. On the other hand, $\Delta S>0$ means the entropy of the system increases. The best indicator of spontaneity in a reaction is the change in Entropy (S or DS). That heat increases the entropy of the surroundings. Although spontaneous reactions are often exothermic, this is not a criteria for spontaneity. Of course this is what is understood as "exothermic".
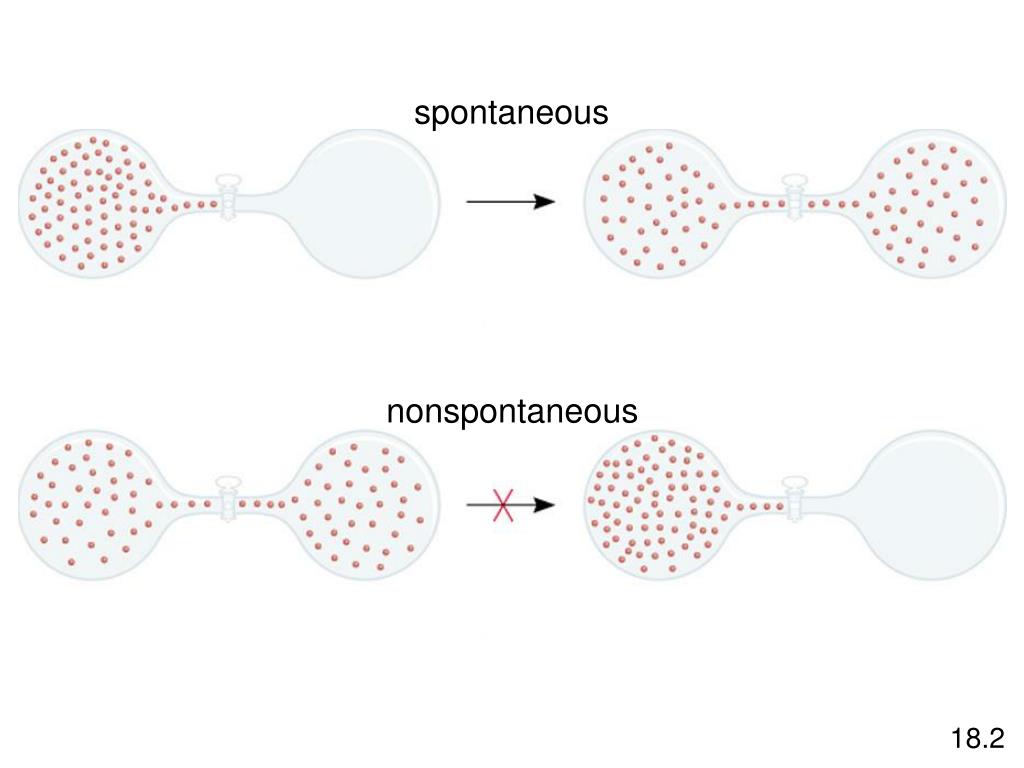
The logical reasoning is as follows: a negative change in enthalpy corresponds at constant P and T to heat being released by the system. Second Law of Thermodynamics (Opens a modal) Work done by isothermic process (Opens a modal) Carnot cycle and Carnot engine (Opens a modal) Proof: Volume ratios in a Carnot cycle. When the process is exothermic (ΔHsystem0), the sign of ΔGsystem is negative at all temperatures. latexDelta Uq+w0+00/latex The spontaneity of this process is therefore not a consequence of any change in energy that accompanies the process. Under these conditions, the above equation suffices to show that what you state in your first paragraph: The first law of thermodynamics confirms that there has been no change in the system’s internal energy as a result of this process. At constant T and P you are then allowed to writeįor the entire range of interest, over which, again, $\Delta S$ and $\Delta H$ are assumed constant. The second assumption is that $\Delta S$ and $\Delta H$, associated with the macroscopic process you are investigating, are independent of T and P over the range of interest. Since you are considering use of the Gibbs free energy as a criterion for spontaneity you are presumably concerned with a process carried out at constant T and P: Considering entropy (S) as a thermodynamic parameter, the criterion for the spontaneity of any process is Here the volume of gas increase from V1 to V2 at. To be able to calculate the temperature at which a process is at equilibrium under standard conditions. Key Learning Objectives To gain an understanding of the relationship between spontaneity, free energy, and temperature. It's a good idea to work out the assumptions implicit in your question. Chemical Thermodynamics Spontaneity: Free Energy and Temperature Jessie A. Spontaneous Process: A process that takes place without any outside influence is called a spontaneous.


 0 kommentar(er)
0 kommentar(er)
